Influence of Water Scale on Thermal Flow Losses of Domestic Appliances
Influence of Water Scale on Thermal Flow Losses of Domestic Appliances
In 2007 an independent scientific study was published in an International Journal proving mineral scale buildup impacts energy efficiency by 10% to 60%; depending on the mineral scale thickness.
Source: International Journal Of Mathematical Models And Methods In Applied Sciences
Author: D. Dobersek and D. Goricanec
Date: February 6, 2007
Download:
In summary:
This scientific study was conducted by D. Dobersek and D. Goricanec in Slovenia to examine if a relationship existed between energy consumption and mineral scale buildup on heating elements.It was found that mineral scale directly impacted energy use by up to 60% because mineral scale reduces the heat conductivity on heat exchanger surfaces; thereby reducing the flow capacity and heat exchange efficiency. This leads to higher investment, operation and maintenance costs as a result of hard water mineral scale.
INTERNATIONAL JOURNAL of MATHEMATICAL MODELS AND METHODS IN APPLIED SCIENCES
Influence of Water Scale on Thermal Flow Losses of Domestic Appliances
D. Dobersek, D. Goricanec
Influence of Water Scale on Thermal Flow Losses of Domestic Appliances
D. Dobersek, D. Goricanec
Abstract - Research results of how the precipitated water scale on heaters of small domestic appliances influences the consumption of electricity are presented. It shows that the majority of water scale samples are composed of aragonite, calcite and dolomite and that those components have an extraordinary low thermal conductivity. Also, the results show that at 2 mm thick deposit, depending on the chemical composition of water scale, the thermal flow is reduced by 10% to 40%; consequently, the consumption of electricity significantly increases.
Key words – Electricity, heat transfer, heat flow, heat exchanger, water scale
I. INTRODUCTIONDUE to increased consumption of drinking and industrial water the whole world is intensively struggling to find new ways of assuring suitable amounts of water of appropriate quality and according to one of the world’s most important thinking how to use energy most efficiently it is reasonable to search the solution for reduction of scale problems [1], [2].
The research is limited to industrial waters which contain a set of dissolved organic and inorganic matters, earths and sands. Larger particles are usually dived with separation methods of mechanical nature. But there is still a problem with dissolved substances in water which, together with microbiological and corrosion products, precipitate in the shape of water scale [3].
These in water dissolved mineral substances that precipitate on walls of flowing water supply systems, on washing machine heaters, in boilers and in dishwashers in a shape of hard linings, cause large technological and economic problems, such as [4], [5]:
Manuscript received February 6, 2007; Revised Received April 15, 2007 D. Dobersek is with University of Maribor, Faculty of Chemistry and Chemical Engineering, Smetanova 17, 2000 Maribor, Slovenia (corresponding author to provide phone: ++386 2 2207 761, e-mail: This email address is being protected from spambots. You need JavaScript enabled to view it.)
D. Goricanec is with University of Maribor, Faculty of Chemistry and Chemical Engineering, Smetanova 17, 2000 Maribor, Slovenia (e-mail: This email address is being protected from spambots. You need JavaScript enabled to view it.)
Issue 2, Volume 1, 2007
Key words – Electricity, heat transfer, heat flow, heat exchanger, water scale
I. INTRODUCTIONDUE to increased consumption of drinking and industrial water the whole world is intensively struggling to find new ways of assuring suitable amounts of water of appropriate quality and according to one of the world’s most important thinking how to use energy most efficiently it is reasonable to search the solution for reduction of scale problems [1], [2].
The research is limited to industrial waters which contain a set of dissolved organic and inorganic matters, earths and sands. Larger particles are usually dived with separation methods of mechanical nature. But there is still a problem with dissolved substances in water which, together with microbiological and corrosion products, precipitate in the shape of water scale [3].
These in water dissolved mineral substances that precipitate on walls of flowing water supply systems, on washing machine heaters, in boilers and in dishwashers in a shape of hard linings, cause large technological and economic problems, such as [4], [5]:
- hindering of water flux in pipes causing eventual congestion;
- reduction of the working volume in heat exchangers and impediment of heat transition;
- a more frequent need for maintenance works;
- a premature replacement of devices.
Manuscript received February 6, 2007; Revised Received April 15, 2007 D. Dobersek is with University of Maribor, Faculty of Chemistry and Chemical Engineering, Smetanova 17, 2000 Maribor, Slovenia (corresponding author to provide phone: ++386 2 2207 761, e-mail: This email address is being protected from spambots. You need JavaScript enabled to view it.)
D. Goricanec is with University of Maribor, Faculty of Chemistry and Chemical Engineering, Smetanova 17, 2000 Maribor, Slovenia (e-mail: This email address is being protected from spambots. You need JavaScript enabled to view it.)
Issue 2, Volume 1, 2007
Additionally, research results of impacts of the abovementioned deposits on thermal transfer reductions for different heat exchangers in households are given, in connection with increased electricity consumption.
II. DRINKING WATER Drinking waters have, depending on their source (ground water, river water, lake or sea water), and also on the season and the ground over and through which they flow, very different compositions and contents of dissolved substances. Beside ions of hardness (Ca2+, Mg2+) waters could contain other cations (Fe2+, Na+, K+) and different amounts of anions (Cl-, SO4 2-, NO3-, HCO3-) [6].
Mainly calcium and magnesium ions influence on the water hardness. The more ions there are dissolved from earth and rocks the hardest the water. Very hard water could be expected in the areas composed of limestone. Water that springs in the areas composed of other rocks and a bit disintegrated silicates is soft just like rainwater.
The classification of water depending on hardness is given in Table I [7].
Water hardness is very different in Slovenia due to various ground compositions. The water is very hard in the area of Kras and at the seaside because of limestone ground. In the area of Pohorje there are mainly old rocks, therefore the water is soft, and in towns of Ljubljana and Maribor the water is middle hard.
TABLE I
WATER HARDNESS IN GERMAN DEGREES
III. WATER SCALE Water scale precipitation is a serious problem in many industrial processes as well in the households, literally everywhere where natural water is used. Supplying waters are usually oversaturated with calcium carbonate. The conditions of over saturation are very often achieved during the operation and that is why the water scale precipitates on heat transfer surfaces in cold water systems as well as in high temperature systems [8].
II. DRINKING WATER Drinking waters have, depending on their source (ground water, river water, lake or sea water), and also on the season and the ground over and through which they flow, very different compositions and contents of dissolved substances. Beside ions of hardness (Ca2+, Mg2+) waters could contain other cations (Fe2+, Na+, K+) and different amounts of anions (Cl-, SO4 2-, NO3-, HCO3-) [6].
Mainly calcium and magnesium ions influence on the water hardness. The more ions there are dissolved from earth and rocks the hardest the water. Very hard water could be expected in the areas composed of limestone. Water that springs in the areas composed of other rocks and a bit disintegrated silicates is soft just like rainwater.
The classification of water depending on hardness is given in Table I [7].
Water hardness is very different in Slovenia due to various ground compositions. The water is very hard in the area of Kras and at the seaside because of limestone ground. In the area of Pohorje there are mainly old rocks, therefore the water is soft, and in towns of Ljubljana and Maribor the water is middle hard.
TABLE I
WATER HARDNESS IN GERMAN DEGREES
| Water classification | Hardness |
| Very soft water | 0 – 4°n |
| Soft water | 4 – 8°n |
| Middle hard water | 8 – 12°n |
| Pretty hard water | 12 – 18°n |
| Hard water | 18 – 30°n |
| Very hard water | > 30°n |
III. WATER SCALE Water scale precipitation is a serious problem in many industrial processes as well in the households, literally everywhere where natural water is used. Supplying waters are usually oversaturated with calcium carbonate. The conditions of over saturation are very often achieved during the operation and that is why the water scale precipitates on heat transfer surfaces in cold water systems as well as in high temperature systems [8].
INTERNATIONAL JOURNAL of MATHEMATICAL MODELS AND METHODS IN APPLIED SCIENCES
A. Precipitation
Scale deposits are mainly composed from calcium carbonate.
In natural waters calcium is presented in ionic form Ca2+. Ca2+ is led to the water as a result of chemical decomposition of calcareous minerals. Ca2+ and CO32- ions are always present in natural water. CO2 from the air is dissolving in water, where a weak carbonic acid is formed [9].
CO2 +H2O ↔H2CO3. (1)
Formed acid than dissociates in two steps:
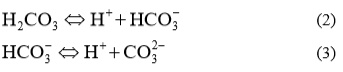
Carbonate ions react with Ca2+ ions and a very slightly soluble calcium carbonate precipitated, according to equation:
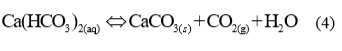
If in balance (4) the concentration of CO2 increase, the balance will moved in left, which means that se CaCO3 will dissolved and pH value will increased. Vice versa by feed the alkalis to the system, the concentration of H+ will reduces, which means, that balance (2) and (3) will move to the right, more CO32- ions will excluded and because of this more solid CaCO3 will precipitated.
CaCO3 starts precipitated, when the concentration of Ca2+ ions is larger from equilibrium. In given moment at creation T and pH the solution is oversaturated. In carbonate balance are Ca2+, CO32-, H+, OH- in HCO3- ions.
From the term of solution electroneutrality, that a charge of all present cations is equal to all present anions, the relationship can be written:
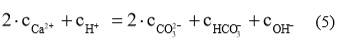
Considering equations (2), (3) in (5) the relationship for Ca2+ concentration in dependence of pH can be written:

The relationship is graphically presented – Fig. 1, from where it is seen that CaCO3 solubility changed with changed temperature. Ta certain value pH solubility starts increasing, because CO32- ions occurs.
Issue 2, Volume 1, 2007
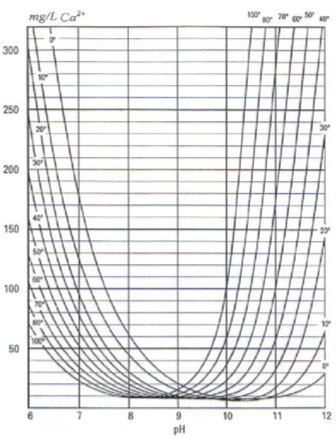
Fig. 1 CaCO3 solubility in ideal solution as a function of pH at certain temperature [9]
If analyse vice versa, it is find that in range pH < 9 solubility falls with increasing temperature. Majority of natural waters are in this range. If pH > 9, the CaCO3 solubility increase with increasing temperature.
B. Crystal structures
The most frequent component of water scale is calcium carbonate, which precipitates in three different forms [10]:
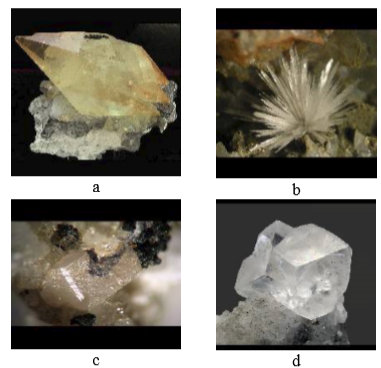
Fig. 2 crystals of calcite (a), aragonite (b), vaterite (c) in dolomite (d) [11]
A. Precipitation
Scale deposits are mainly composed from calcium carbonate.
In natural waters calcium is presented in ionic form Ca2+. Ca2+ is led to the water as a result of chemical decomposition of calcareous minerals. Ca2+ and CO32- ions are always present in natural water. CO2 from the air is dissolving in water, where a weak carbonic acid is formed [9].
CO2 +H2O ↔H2CO3. (1)
Formed acid than dissociates in two steps:
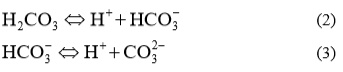
Carbonate ions react with Ca2+ ions and a very slightly soluble calcium carbonate precipitated, according to equation:
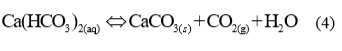
If in balance (4) the concentration of CO2 increase, the balance will moved in left, which means that se CaCO3 will dissolved and pH value will increased. Vice versa by feed the alkalis to the system, the concentration of H+ will reduces, which means, that balance (2) and (3) will move to the right, more CO32- ions will excluded and because of this more solid CaCO3 will precipitated.
CaCO3 starts precipitated, when the concentration of Ca2+ ions is larger from equilibrium. In given moment at creation T and pH the solution is oversaturated. In carbonate balance are Ca2+, CO32-, H+, OH- in HCO3- ions.
From the term of solution electroneutrality, that a charge of all present cations is equal to all present anions, the relationship can be written:
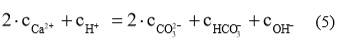
Considering equations (2), (3) in (5) the relationship for Ca2+ concentration in dependence of pH can be written:

The relationship is graphically presented – Fig. 1, from where it is seen that CaCO3 solubility changed with changed temperature. Ta certain value pH solubility starts increasing, because CO32- ions occurs.
Issue 2, Volume 1, 2007

Fig. 1 CaCO3 solubility in ideal solution as a function of pH at certain temperature [9]
If analyse vice versa, it is find that in range pH < 9 solubility falls with increasing temperature. Majority of natural waters are in this range. If pH > 9, the CaCO3 solubility increase with increasing temperature.
B. Crystal structures
The most frequent component of water scale is calcium carbonate, which precipitates in three different forms [10]:
- calcite (Fig.2a)
- aragonite (Fig. 2b)
- vaterite (Fig. 2c)

Fig. 2 crystals of calcite (a), aragonite (b), vaterite (c) in dolomite (d) [11]
INTERNATIONAL JOURNAL of MATHEMATICAL MODELS AND METHODS IN APPLIED SCIENCES
Their stability is falling round listed order. Very often aragonite precipitated first, more rarely vaterit, which than crystallise into calcite. Fig. 3 presents the solubility of individual calcium carbonate shapes in dependence of temperature.
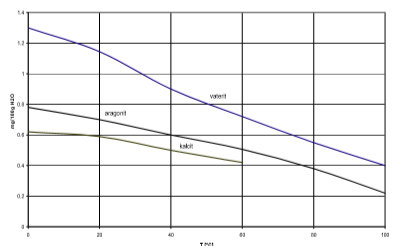
Fig. 3 solubility of different crystal shapes of calcium carbonate
Calcite crystals are usually white or without colours and have a rhombohedra crystal structure. The density of calcite is 2.7 g/cm3 and its thermal conductivity 2.72 W/mK at 100°C. Rhombohedra crystals have large connection areas and that is why the linings are more compact.
On the other hand we have needles aragonite crystals with smaller contact areas and less compact linings.
Vaterite crystals with hexagonal structure rarely occur and have a density of 2.6 g/cm3.
Others bivalent ions which are in water usually precipitate in smaller quantities and indirectly effect the precipitation of calcium carbonate.
At the conditions of calcium carbonate precipitation from natural waters the solubility of magnesium carbonate is not exceeded, however Mg2+ ions are built into calcite and a new predominant carbonate solid phase of dolomite is formed (Fig. 2 d). Thermal conductivity of dolomite is also very low at 4.78 W/mK at 100°C, and its density is 2.8 g/cm3.
IV. ELECTRICITY CONSUMPTION OF A SMALL DOMESTIC APPLIANCE According to statistical data the amount of average electricity consumption in a Slovene household is 290 kWh per month. However, this data does not say enough because the consumption depends on the size of the household, the number of electrical machines and the quality and intensity of their use – as shown in Table II [12].
The use of electricity of electrical boiler for warm water or washing machine and dishwasher could rise due to water scale deposit on heat exchangers. The amount of exceeded water scale depends on water composition, operational temperature and the type and roughness of the material.
Issue 2, Volume 1, 2007

Fig. 3 solubility of different crystal shapes of calcium carbonate
Calcite crystals are usually white or without colours and have a rhombohedra crystal structure. The density of calcite is 2.7 g/cm3 and its thermal conductivity 2.72 W/mK at 100°C. Rhombohedra crystals have large connection areas and that is why the linings are more compact.
On the other hand we have needles aragonite crystals with smaller contact areas and less compact linings.
Vaterite crystals with hexagonal structure rarely occur and have a density of 2.6 g/cm3.
Others bivalent ions which are in water usually precipitate in smaller quantities and indirectly effect the precipitation of calcium carbonate.
At the conditions of calcium carbonate precipitation from natural waters the solubility of magnesium carbonate is not exceeded, however Mg2+ ions are built into calcite and a new predominant carbonate solid phase of dolomite is formed (Fig. 2 d). Thermal conductivity of dolomite is also very low at 4.78 W/mK at 100°C, and its density is 2.8 g/cm3.
IV. ELECTRICITY CONSUMPTION OF A SMALL DOMESTIC APPLIANCE According to statistical data the amount of average electricity consumption in a Slovene household is 290 kWh per month. However, this data does not say enough because the consumption depends on the size of the household, the number of electrical machines and the quality and intensity of their use – as shown in Table II [12].
The use of electricity of electrical boiler for warm water or washing machine and dishwasher could rise due to water scale deposit on heat exchangers. The amount of exceeded water scale depends on water composition, operational temperature and the type and roughness of the material.
Issue 2, Volume 1, 2007
TABLE II ANNUAL ELECTRICITY CONSUMPTION OF A SMALL DOMESTIC APPLIANCE
V. REDUCED HEAT TRANSFER BECAUSE OF THE WATER SCALEThe water scale formed on the surfaces of heat exchangers has very low heat conductivity, it reduces the flow capacity and heat exchange efficiency, leading to a higher investment, operation and maintenance costs.
Two types of heat exchangers are used in practice for heat transfer in water [13]:
A. Plate heat exchanger
If the heat exchanger is described as a flat wall with area A (Fig. 3), then the heat flow intensity is determined by equation (7) and equation (8) respectively:
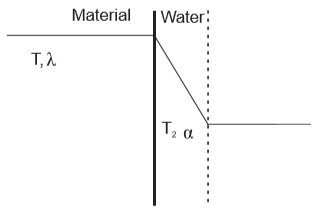
Fig. 3 the temperature curve through the heat exchanger wall
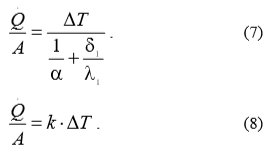
Where:
Q = heat flow
A = heat exchanger area
| Domestic appliance | Electricity use per household (kWh/year) |
| Electrical boiler | 1080 |
| Washing machine | 300 |
| Dishwasher | 410 |
| Dryer | 150 |
| Electrical kitchen-range | 600 |
| Refrigerator and freezer | 960 |
| Lights | 310 |
V. REDUCED HEAT TRANSFER BECAUSE OF THE WATER SCALEThe water scale formed on the surfaces of heat exchangers has very low heat conductivity, it reduces the flow capacity and heat exchange efficiency, leading to a higher investment, operation and maintenance costs.
Two types of heat exchangers are used in practice for heat transfer in water [13]:
- plate heat exchanger
- pipe heat exchanger.
A. Plate heat exchanger
If the heat exchanger is described as a flat wall with area A (Fig. 3), then the heat flow intensity is determined by equation (7) and equation (8) respectively:

Fig. 3 the temperature curve through the heat exchanger wall
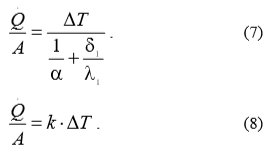
Where:
Q = heat flow
A = heat exchanger area
INTERNATIONAL JOURNAL of MATHEMATICAL MODELS AND METHODS IN APPLIED SCIENCES
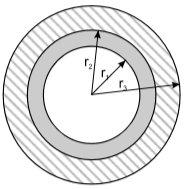
Fig. 6 pipe heat exchanger
Because water scale reduces heat transfer the equation (12) becomes equation (13):
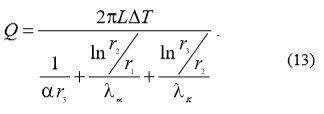
With the use of equations (12) and (13) equation (14) can be written which enables the calculation of heat transfer drop ξ:
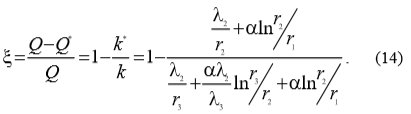
C. Heat losses caused by the composition of linings
With the view of investigating the phenomena of scale formation, the causes of intensity of scale precipitation on washing machine heaters and on heaters of boilers for hot water at different operating conditions were examined. Crucial parameters for the conducted experiments were water hardness, the temperature of operation. On Fig. 7 heaters from washing machine are presented after 300 cycles of washing at 60°C and 95°C without washing powders.
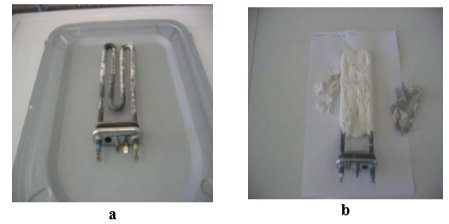
Fig. 7 washing machine heaters after 300 cycles of washing without washing powders (a – at 60°C, b – at 95°C)
ΔT temperature difference
α heat conversion
δ1 heat exchanger wall thickness
λ1 heat conductivity
k heat transition
The water scale on heat exchanger (Fig. 4) reduces the heat transfer that is why equations (7) and (8) become equations (9) and (10):
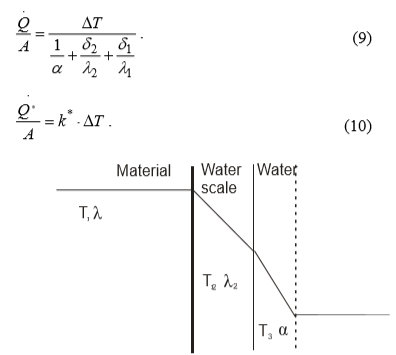
Fig. 4 the temperature curve through the heat exchanger wall and water scale
With the equations (7) and (9) the equation (11) can be written which enables the calculation of heat transfer drop ξ, because of water scale linings:
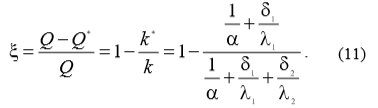
B. Pipe heat exchanger
If the heat exchanger has the shape of a pipe with inner diameter rn (Fig. 5), then the heat flow through the pipe wall is given with the following equation (12):
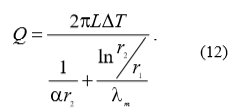
Where:
L pipe length
r2 external pipe diameter
r1 internal pipe diameter
Issue 2, Volume 1, 2007
α heat conversion
δ1 heat exchanger wall thickness
λ1 heat conductivity
k heat transition
The water scale on heat exchanger (Fig. 4) reduces the heat transfer that is why equations (7) and (8) become equations (9) and (10):

Fig. 4 the temperature curve through the heat exchanger wall and water scale
With the equations (7) and (9) the equation (11) can be written which enables the calculation of heat transfer drop ξ, because of water scale linings:
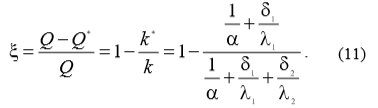
B. Pipe heat exchanger
If the heat exchanger has the shape of a pipe with inner diameter rn (Fig. 5), then the heat flow through the pipe wall is given with the following equation (12):
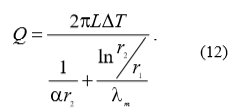
Where:
L pipe length
r2 external pipe diameter
r1 internal pipe diameter
Issue 2, Volume 1, 2007

Fig. 6 pipe heat exchanger
Because water scale reduces heat transfer the equation (12) becomes equation (13):
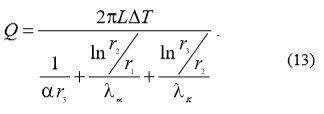
With the use of equations (12) and (13) equation (14) can be written which enables the calculation of heat transfer drop ξ:
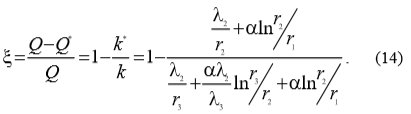
C. Heat losses caused by the composition of linings
With the view of investigating the phenomena of scale formation, the causes of intensity of scale precipitation on washing machine heaters and on heaters of boilers for hot water at different operating conditions were examined. Crucial parameters for the conducted experiments were water hardness, the temperature of operation. On Fig. 7 heaters from washing machine are presented after 300 cycles of washing at 60°C and 95°C without washing powders.

Fig. 7 washing machine heaters after 300 cycles of washing without washing powders (a – at 60°C, b – at 95°C)
INTERNATIONAL JOURNAL of MATHEMATICAL MODELS AND METHODS IN APPLIED SCIENCES
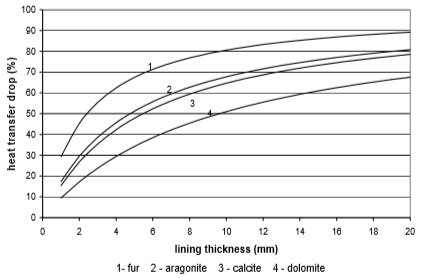
Fig. 9 Heat transfer through copper plate heat exchanger
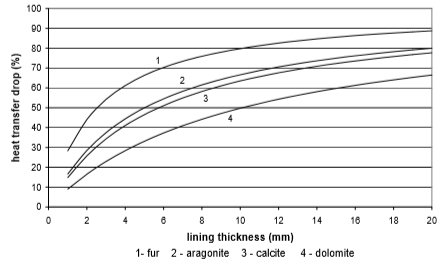
Fig. 10 Heat transfer through stainless steel plate heat exchanger
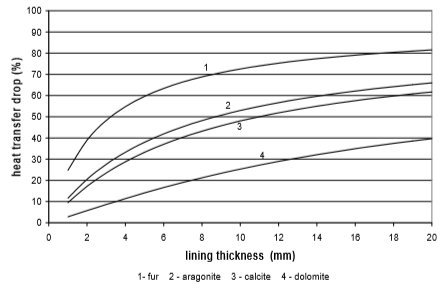
Fig. 11 Heat transfer through the copper pipe
On Fig. 8 the outlet pipes from the boiler for hot water after 14 days continuous run are presented.
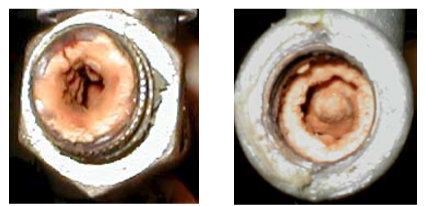
Fig. 8 the outlet pipes from the boiler for hot water
To evaluate the heat transfer reduction caused by water scale linings, the chemical structure of linings and the material of heat exchanger have to be known. Most often the materials used for heat exchangers are [14]:
Thermal conductivity values for components which most often occur in water scale are given in table III.
TABLE III
THERMAL CONDUCTIVITIES OF WATER SCALE COMPONENTS
The data needed for the calculation of reduced thermal efficiency caused by water scale linings, for the example from chapter A. are:
The data for calculation of reduced thermal efficiency due to deposit of water scale for the case from chapter B. are:
The use of electricity depending on water scale thickness, in relation to the chemical structure of deposit and the type of heat exchanger, is calculated on the basis of values from Fig. 9 to 12. Results are given in Fig. 13 to 15

Fig. 8 the outlet pipes from the boiler for hot water
To evaluate the heat transfer reduction caused by water scale linings, the chemical structure of linings and the material of heat exchanger have to be known. Most often the materials used for heat exchangers are [14]:
- copper with thermal conductivity of 395 W/mK;
- stainless steal with thermal conductivity of 26 W/mK.
Thermal conductivity values for components which most often occur in water scale are given in table III.
TABLE III
THERMAL CONDUCTIVITIES OF WATER SCALE COMPONENTS
| Component | Thermal conductivity |
| Aragonite | 2.37 W/mK |
| Calcite | 2.72 W/mK |
| Dolomite | 4.78 W/mK |
| Fur | 1.2 W/mK |
The data needed for the calculation of reduced thermal efficiency caused by water scale linings, for the example from chapter A. are:
- convection coefficient 500 W/m2K; and
- heat exchanger thickness 3mm.
The data for calculation of reduced thermal efficiency due to deposit of water scale for the case from chapter B. are:
- convection coefficient 500 W/m2K;
- internal pipe diameter 10 mm;
- external pipe diameter 13 mm.
The use of electricity depending on water scale thickness, in relation to the chemical structure of deposit and the type of heat exchanger, is calculated on the basis of values from Fig. 9 to 12. Results are given in Fig. 13 to 15

Fig. 9 Heat transfer through copper plate heat exchanger

Fig. 10 Heat transfer through stainless steel plate heat exchanger

Fig. 11 Heat transfer through the copper pipe
INTERNATIONAL JOURNAL of MATHEMATICAL MODELS AND METHODS IN APPLIED SCIENCES
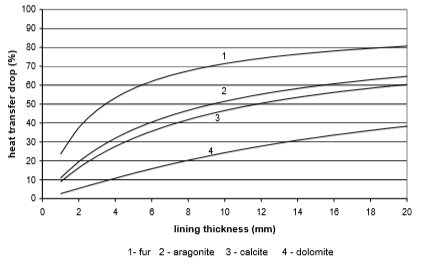
Fig. 12 Heat transfer through the stainless steal pipe
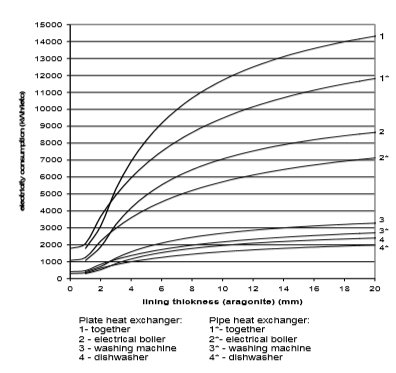
Fig. 13 Electricity consumption for plate and pipe heat exchanger in the case of aragonite
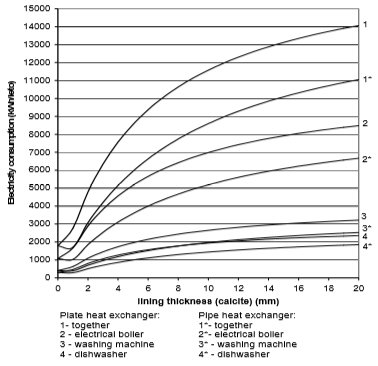
Fig. 14 Electricity consumption for plate and pipe heat exchanger in the case of calcite
Issue 2, Volume 1, 2007
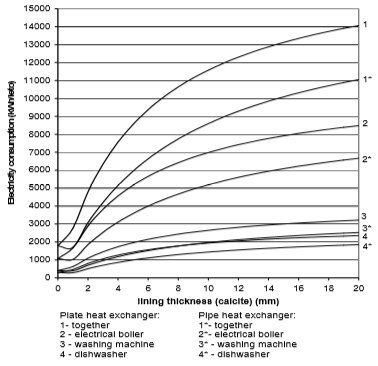
Fig. 15 Electricity consumption for plate and pipe heat exchanger in the case of fur
VI. DISCUSSION AND CONCLUSION Water scale causes a lot of problems of heat transfer due to its extremely low thermal conductivity. Water scale is most frequently composed of dolomite and calcium carbonate, which precipitate as calcite and aragonite. Rarely calcium sulphate and magnesium carbonate precipitate. A common name for above-mentioned components is fur, which has a thermal conductivity of 1.2 W/mK.
From the Figures 10 to 13 it can be seen how the lining of water scale reduces heat transfer. If the linings are around 3 mm thick, the heat transfer is reduced for 25% to 60%, in the case of a plate heat exchanger, made from cooper or stainless steal.
A bit better results are achieved for pipe heat exchangers where at the same thickness the heat transfer is reduced for 10% to 50%. Through the research the amount of precipitated water scale on heat exchangers of washing machine and boiler was measured. It was found out that after 300 washing cycles in washing machine at 95°C the linings are 15 mm thick. Linings were thinner in the boiler and dishwasher, and were 3 to 5 mm thick in average.
If we assume that each of the 670,000 households in Slovenia has one washing machine, one boiler and one dishwasher, then around 10,000 kWh more of electricity will be used per year due to water scale precipitation. The electrical boiler will in average use 6,000 to 8,000 kWh of electricity per household per year, washing machine 1,000 to 1100 kWh/household/year, and dishwasher 1,500 to 1,700 kWh/household/year.

Fig. 12 Heat transfer through the stainless steal pipe

Fig. 13 Electricity consumption for plate and pipe heat exchanger in the case of aragonite

Fig. 14 Electricity consumption for plate and pipe heat exchanger in the case of calcite
Issue 2, Volume 1, 2007

Fig. 15 Electricity consumption for plate and pipe heat exchanger in the case of fur
VI. DISCUSSION AND CONCLUSION Water scale causes a lot of problems of heat transfer due to its extremely low thermal conductivity. Water scale is most frequently composed of dolomite and calcium carbonate, which precipitate as calcite and aragonite. Rarely calcium sulphate and magnesium carbonate precipitate. A common name for above-mentioned components is fur, which has a thermal conductivity of 1.2 W/mK.
From the Figures 10 to 13 it can be seen how the lining of water scale reduces heat transfer. If the linings are around 3 mm thick, the heat transfer is reduced for 25% to 60%, in the case of a plate heat exchanger, made from cooper or stainless steal.
A bit better results are achieved for pipe heat exchangers where at the same thickness the heat transfer is reduced for 10% to 50%. Through the research the amount of precipitated water scale on heat exchangers of washing machine and boiler was measured. It was found out that after 300 washing cycles in washing machine at 95°C the linings are 15 mm thick. Linings were thinner in the boiler and dishwasher, and were 3 to 5 mm thick in average.
If we assume that each of the 670,000 households in Slovenia has one washing machine, one boiler and one dishwasher, then around 10,000 kWh more of electricity will be used per year due to water scale precipitation. The electrical boiler will in average use 6,000 to 8,000 kWh of electricity per household per year, washing machine 1,000 to 1100 kWh/household/year, and dishwasher 1,500 to 1,700 kWh/household/year.
INTERNATIONAL JOURNAL of MATHEMATICAL MODELS AND METHODS IN APPLIED SCIENCES
REFERENCES
REFERENCES
[1] D. Dobersek, D. Goricanec, J. Krope, “Economic analysis of energy savings by using rotary heat regenerator in ventilating systems,” IASME Trans. vol. 4, pp..1640-1647, 2005.
[2] J. Kropej, D. Dobersek, D. Goricanec, “Economic evaluation of possible use of heat of flue gases in a heating plant,” WSEAS transactions on heat and mass transfer, vol. 1, pp. 75-80 2006.
[3] C. A. J. Appelo, D. Postma, Geochemistry groundwater and pollution, CRC Press, 1993
[4] L. Lipus, D. Dobersek, “Influence of magnetic field on the aragonite precipitation, “ Chem. eng. sci., Apr., vol. 62, pp. 2089-2095, 2007.
[5] D. Dobersek, D. Goricanec, J. Krope, “The influence of physico - chemical parameters on water scale precipitation on washing machines heaters, “ Acta chim. Slov,. vol. 54, pp. 719-724, 2007.
[6] W. Stumm, Aquatic Chemistry - Chemical Equilibria and Rates in Natural Waters, A Wiley – Inter. Pub.,1996.
[2] J. Kropej, D. Dobersek, D. Goricanec, “Economic evaluation of possible use of heat of flue gases in a heating plant,” WSEAS transactions on heat and mass transfer, vol. 1, pp. 75-80 2006.
[3] C. A. J. Appelo, D. Postma, Geochemistry groundwater and pollution, CRC Press, 1993
[4] L. Lipus, D. Dobersek, “Influence of magnetic field on the aragonite precipitation, “ Chem. eng. sci., Apr., vol. 62, pp. 2089-2095, 2007.
[5] D. Dobersek, D. Goricanec, J. Krope, “The influence of physico - chemical parameters on water scale precipitation on washing machines heaters, “ Acta chim. Slov,. vol. 54, pp. 719-724, 2007.
[6] W. Stumm, Aquatic Chemistry - Chemical Equilibria and Rates in Natural Waters, A Wiley – Inter. Pub.,1996.
[7] J. H. Lehr, Water Encyclopedia: Domestic, Municipal, and Industrial Water Supply and Waste Disposal, John Wiley&Sons, 2005.
[8] M.M. Reddy, G.H. Nancollas, “Crystalization of calcium carbonate, Isotopic exchange and kinetics, “ Journal of Crystal Growth, 35, pp. 33 – 38, 1976.
[9] H. Kristiansen, “The Calcium Concentration at Different Temperatures as Function of the pH for Water in a Carbonate Equilibrium, “ WATTEN 1pp. 7-15.8, 1975.
[10] O. Johnsen, Photographic guide to minerals of the world, Oxford university press, 2002
[11] http://www.mindat.org/min-859.html
[12] Statistical office of the Republic of Slovenia.
[13] J. P. Holman, Heat transfer, Mc-Grew Hill, 1986.
[14] D. L. Ride, Handbook of Chemistry and Physics,CRC Press, 2007
[8] M.M. Reddy, G.H. Nancollas, “Crystalization of calcium carbonate, Isotopic exchange and kinetics, “ Journal of Crystal Growth, 35, pp. 33 – 38, 1976.
[9] H. Kristiansen, “The Calcium Concentration at Different Temperatures as Function of the pH for Water in a Carbonate Equilibrium, “ WATTEN 1pp. 7-15.8, 1975.
[10] O. Johnsen, Photographic guide to minerals of the world, Oxford university press, 2002
[11] http://www.mindat.org/min-859.html
[12] Statistical office of the Republic of Slovenia.
[13] J. P. Holman, Heat transfer, Mc-Grew Hill, 1986.
[14] D. L. Ride, Handbook of Chemistry and Physics,CRC Press, 2007